
Winter air quality can be worse than in summer. Here's why
Stagnant winter weather can produce smoggy conditions that can rival any summer day.
Smog is typically seen as a summer problem, but winter air quality can be just as bad or even worse.
Thoughts of smoggy days often conjure images of sweltering heat and blazing sunlight. This is understandable since the major components of smog — ozone and fine particulate matter — are photochemical pollutants. That is, they form in the presence of sunlight, and the more intense the sunlight is, the higher the pollutant concentrations will climb. Thus, smog episodes tend to happen in the warmer months of the year, from late spring through early fall.
However, as we saw over New Year's, Environment and Climate Change Canada issued a smog warning due to poor air quality. According to CBC News, the warning applied to Montreal, areas along the St. Lawrence river up to the northeast of Quebec City, as well as the Beauce region, south of Quebec City, and in Gatineau, near Ottawa. Residents of Montreal were advised not to use wood-burning heating appliances while the smog warning was in effect.
So, what happened?
THE DOSE MAKES THE POISON
The higher the concentration of an air pollutant, the more potential harm it can cause. When checking your local air quality report, you will often see terms like "parts per million" (ppm), or "parts per billion" (ppb), or "micrograms per cubic metre" (μg/m³). These represent the concentration — how much of the pollutant is found in a specific amount of air.
There are three basic ways to increase the concentration of an air pollutant. The first is the most obvious: with the volume of air staying the same, the actual physical amount of pollutants increases. The second way is to have the same amount of pollutants present but a smaller volume of air available for those pollutants to mix into. The third, and most hazardous to our health, is a combination of both — more pollution mixing into a reduced volume of air.

Smog inundates Los Angeles, while clear skies are seen above the city. Credit: Getty Images
There are several ways that we can see more pollution in the air. For example, put tens of thousands of cars on the road during Toronto's rush hours, or have the winds shift so that emissions from the dozens of coal-fired powerplants in the U.S. Midwest are carried into the region, or have a day of stagnant weather with no wind to carry pollutants away from urban centres. These are just a few examples of when we could have elevated air pollution concentrations.
On the other hand, shrinking the volume of air available for pollutants to mix into is almost always a result of one particular meteorological phenomenon — the temperature inversion.
THE DREADED 'INVERSION'
Temperatures in the atmosphere change with height. In the troposphere — the lowest level of the atmosphere, where nearly all of our weather occurs — we tend to find the warmest air nearest to the ground, and the temperature gradually gets colder the higher you go.
However, this typical pattern can be disrupted by the presence of a layer of warmer air high above the ground. This produces a temperature inversion.
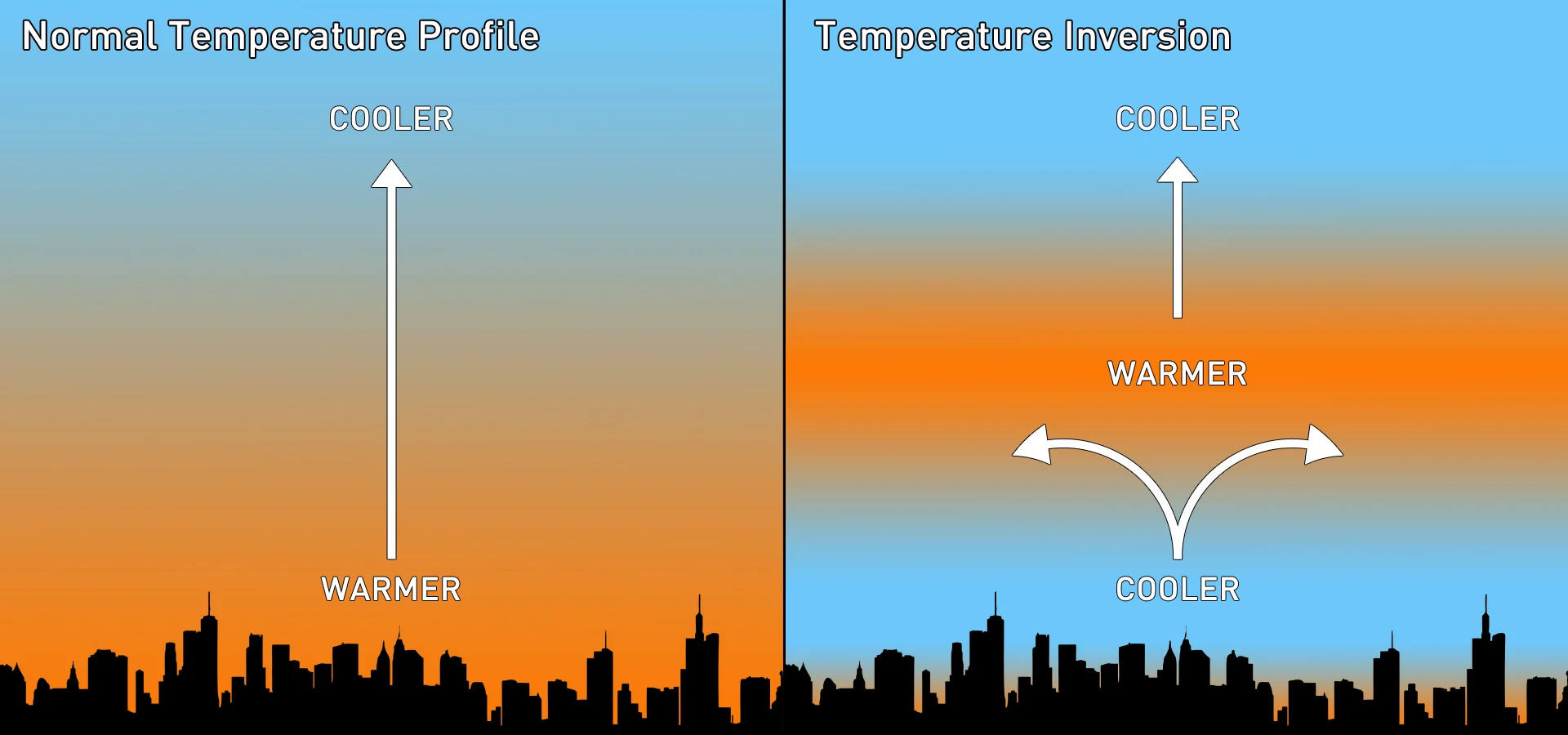
The normal temperature profile of the troposphere (left) and the temperature profile with an inversion present (right). Credit: Scott Sutherland
When there's a temperature inversion, the warm air aloft basically acts as a barrier. As a result, any rising parcels of air are stopped and can only spread out along the base of the warm layer. Typically, the troposphere's depth is around 12 kilometres. An inversion can form just half a kilometre above the ground, severely restricting the volume of air available for pollutants to mix into.
Whether it's car exhaust, industrial emissions, or smoke from woodburning stoves and fireplaces, pollution that gets trapped under an inversion can become very concentrated in just a short amount of time.
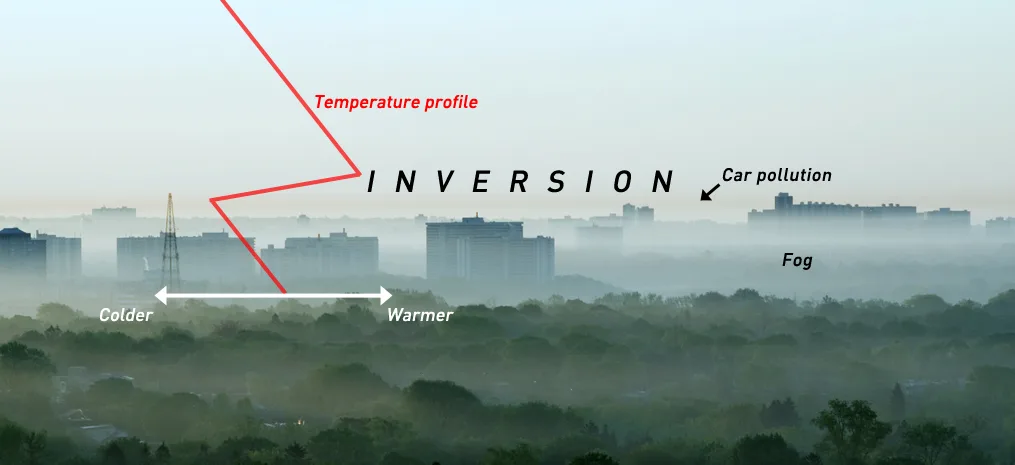
This image from Toronto shows a classic summer inversion. The labels highlight what we're seeing. The temperature profile reveals the inversion, the layer of brown car emissions trapped directly under the inversion, and the morning fog blanketing this part of the city. Credit: Getty Images/Scott Sutherland
Most often, temperature inversions form in still weather during overnight hours. As the ground quickly cools at night, it absorbs heat from the air above it. If the air is too warm to completely cool by this process, by morning, the result will be a layer of colder air near the surface, with warmer air higher up. This can happen at any time of year. However, the strongest inversions usually occur in the winter due to the longer nights and colder ground.
Inversions can also form under domes of high pressure, which feature air sinking towards the ground from above. As that air descends, it warms as it draws closer to the ground. When the sinking air parcels from above meet rising air parcels from the ground with the same temperature, they stop and mix, resulting in a stable inversion.
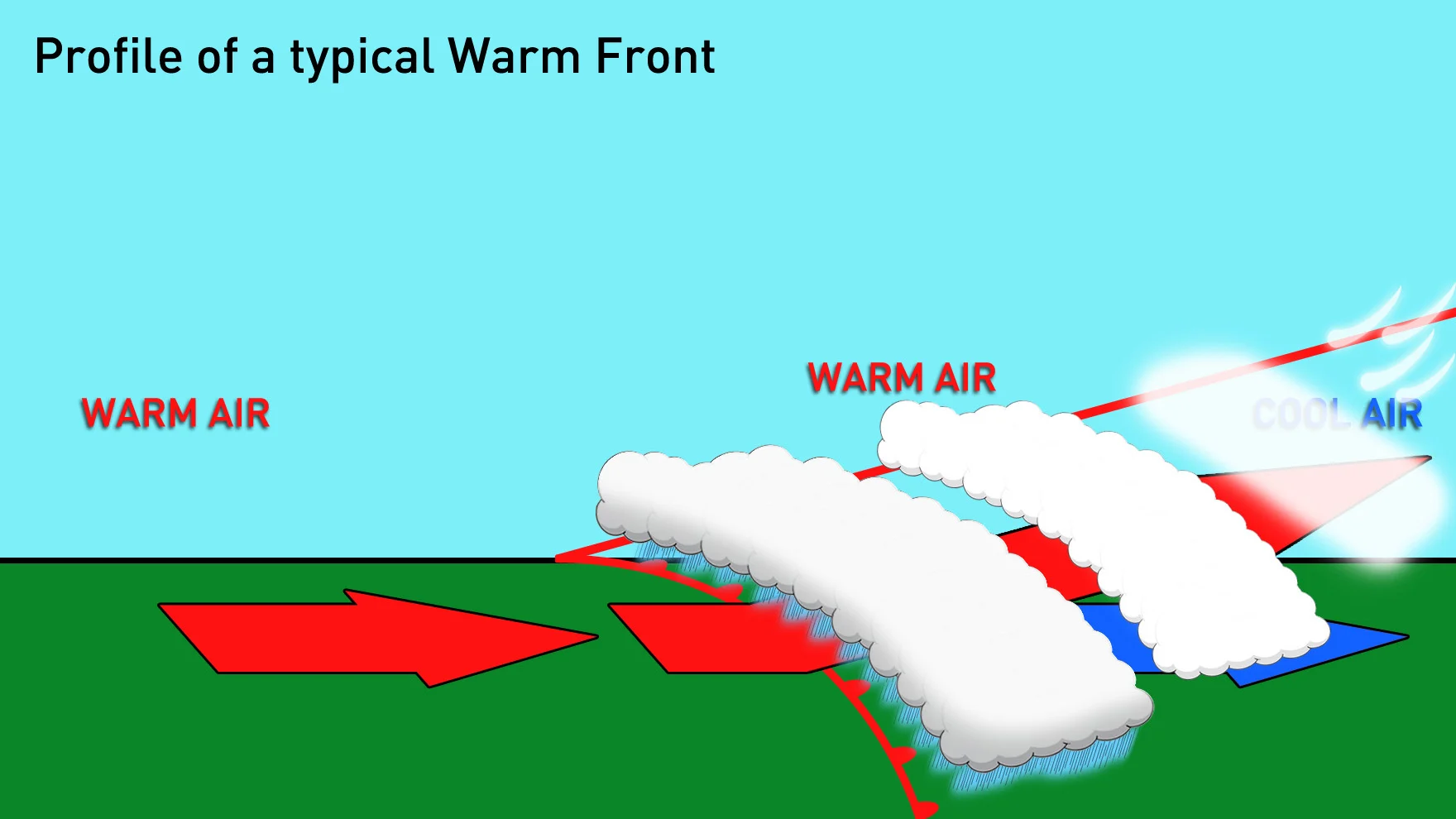
This Weather Wise graphic shows a 3D perspective of a warm front. Credit: Scott Sutherland
As shown above, inversions are also associated with warm fronts. Warm, humid air spiralling into a low pressure system rides up over the cooler, denser air the system is pushing into. The presence of a frontal inversion is how we get precipitation such as snow pellets, ice pellets, and freezing rain.
Inversions are usually short-lived. The conditions move along with the prevailing weather, so they tend to only persist over a region for a few days. Even under the same conditions, if the air below an inversion warms up sufficiently throughout the day, this can 'break' the inversion. Valley inversions — like those we see in the B.C. Interior — can be particularly tenacious.
Weather Network meteorologist Erin Wenckstern explains how these form, in the video below:
BRIGHT, SNOWY OZONE DAYS
Ground-level ozone is usually a problem in the warmer part of the year. However, there is a very specific exception to this that can occur in winter.
As mentioned above, ozone is a photochemical. Thus, intense sunlight is required to produce high concentrations of it. On a bright, sunny winter day, with stagnant winds and a temperature inversion, the weather conditions are primed for a high ozone day, the same as they would be during the summer. However, with the Sun lower in the winter sky, sunlight loses much of its intensity by the time it reaches the ground. Therefore, while other pollutants may become more concentrated, ozone levels will likely remain low.
One additional factor can change this, though. Add a layer of clean, freshly fallen snow or a layer of smooth ice over snow, and it can result in a very interesting situation.
Normally, incoming photons of sunlight interact with the pollution in the air just once. Any photons that make it through the polluted air are then absorbed by the ground. However, suppose the ground is covered by a fresh blanket of snow or a layer of smooth ice over white snow. In that case, this substantially increases the ground's albedo. Thus, a significant number of the photons that make it through the layer of polluted air are immediately reflected back up for a second pass, with little or no loss of intensity.

Fresh snow significantly increases the albedo of the ground during this stunning display of atmospheric optics, captured at Petite-Riviere-Saint-Francois, QC, on January 4, 2021. Credit: Dominique Jacques-Brissette
This can result in up to a double-dose of ozone production, potentially turning a low or moderate risk AQHI day into one where ozone levels rise to be high risk.
WHERE TO CHECK THE AQHI
At any time of the year, you can check the current AQHI value for your community on in a few different places. It is listed on your local city page at theweathernetwork.com.
It is also available from The Weather Network app or from the Environment Canada website.
